Abstract
Ibrutinib is a first-in-class, once-daily, oral irreversible inhibitor of the Bruton's tyrosine kinase (BTK). This agent is approved for treating adult patients (pts) with chronic lymphocytic leukemia (CLL). In multiple phase 3 trials with previously untreated or relapsed/refractory (R/R) CLL pts, ibrutinib demonstrated significant benefits in progression-free survival (PFS) and overall survival (OS) with a favorable tolerability profile [1-5].
EVIDENCE is a prospective, multicenter, non-interventional real world (RW) study that investigated the retention rate (RR), effectiveness, and safety of ibrutinib given as 1st, 2nd, or later lines of treatment in pts with CLL during a 2-year-time of clinical observation period.
This study included 309 pts from 39 Italian centers who were treated with ibrutinib between Nov. 2018 and Oct. 2019. Ibrutinib was given in 118 pts as 1stline treatment (1L), in 127 pts as 2nd line treatment (2L), and in 64 pts as >2L treatment.
The primary endpoint was the RR (defined as the percentage between the number of pts on ibrutinib at 24 months over the number of pts who had discontinued treatment at risk) and the secondary endpoints were: PFS, OS, Time To Next Treatment (TTNT) and safety.
The median age of pts at baseline was 71.3 years (yrs) for treatment-naïve (TN) pts treated in 1L (TN cohort C1), 68.7 yrs for pts in 2L (cohort C2), and 70.7 yrs in those at later lines (cohort C3).The Eastern Cooperative Oncology Group (ECOG) performance status was 0-1 in 77.0% of pts. TP53 aberration (del17p and/or TP53 mutation) was detected in 53.3% of tested pts in C1, and in 41.8% and 60.9% of pts in the C2 and C3, respectively. Unmutated IGHV was 31.7%, 38.2% and 30.4% in C1, C2, and C3, respectively.
Overall, 63.1% of pts showed one or more comorbidities at baseline. A cardiovascular (CV) disorder was present in 33.3% of pts, 46% of pts were on antihypertensive medications and 17.5% on anticoagulants and/or antiplatelet agents.
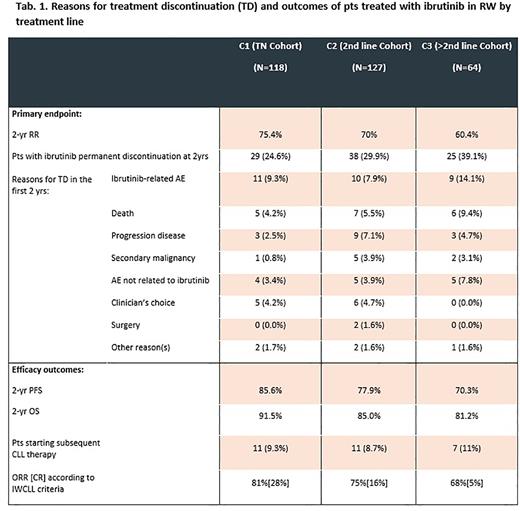
The majority of pts remained on ibrutinib treatment after 2 yrs. The 2-yr RR was 75.4% in C1, 70.0% in C2, and 60.4% in C3 cohort. Discontinuation due to ibrutinib-related AEs in the first 2 yrs occurred in 9.3%, 7.9% and 14.1% in C1, C2 and C3 respectively. Discontinuation due to progression disease (PD) or deaths in the first 2 yrs occurred in 6.7%, 12.6%, 14.1% in the C1, C2 and C3, respectively.
In the multivariate analysis: age (70 vs <70 yrs), gender, Binet stage (A vs B/C), CVD (present vs absent), and treatment line (TN vs C2/C3) did not show a significant impact on the RR, while the number of Adverse Drug Reactions (ADRs) [0 vs. 1/≥2] emerged as the only independent factor impacting RR.
The 2-yr PFS was 85.6%, 77.9%, and 70.3% in C1, C2, and C3, respectively (Tab. 1). The 2-yr OS was 91.5%, 85%, and 81.2% in C1, C2, and C3, respectively (Tab. 1). The 2-yrs TTNT was not reached in all treatment cohorts.
Cumulative incidence of non-hematological AEs was lower than reported in clinical trials, in particular any grade bleeding events were reported in 13.6% pts in C1, 10.2% and 17.2% pts in C2 and C3 respectively. Incidence of any grade of atrial fibrillation (AF) was 7.6% in C1, 8.7% in C2 and 9.4% in C3, while grade 3-4 of 2.5% in C1, and 3.9% and 6.3% in C2 and C3 respectively. Any grade infection was 23.7% in C1 and 26.8% and 31.3% in C2 and C3 respectively. Any grade hypertension (HTN) was 2.5% in C1 and 3.1% and 9.4% in C2 and C3 respectively. No new safety signals were observed.
In this RW study the 2-yr RR were the highest among pts who received ibrutinib as 1L. Among ibrutinib-related AE infectious events were the most frequent cause of TD, accounting for 4.5%. Rate of CV events including AF, bleedings and HTN wasn't higher than reported in clinical trials and according to previous RW studies [6-8].Therefore an appropriate screening at baseline, management of CV complications and infections prophylaxis could improve treatment adherence of RW CLL pts treated with ibrutinib. Efficacy data and safety are consistent with other clinical trials.
References: 1. Byrd, JC, et al. N Engl J Med. 2014; 371:213-23;
2. O'Brien, S, et al. Blood. 2018; 131:1910-9;
3. Burger, JA, et al. Leukemia. 2020; 34(3):787-798. 18.
4. Shanafelt TD, et al N Engl J Med . 2019;381:432-443.
5. Woyach JA, et al. N Engl J Med 2018. 20.
6. Aarup K, et al. Eur J Haematol . 2020.
7. Salles G, et al. Ann Hematol. 2019;98:2749-2760.
8. Pau Abrisqueta et al. Clinical Lymphoma, Myeloma and Leukemia 2021.
Disclosures
Molica:AbbVie, Janssen, Astra-Zeneca: Consultancy, Honoraria. Scarfò:Octopharma: Speakers Bureau; BeiGene, Janssen: Other: Travel Grant; AbbVie, AstraZeneca, Janssen, Beigene: Honoraria. Murru:Janssen: Research Funding; Janssen, Abbvie, Astra Zeneca: Honoraria, Other: travel. Frigeri:Janssen, Abbvie, GSK, Celgene, EUSA Pharma: Consultancy. Sanna:Janssen: Consultancy, Honoraria, Other: travel, Speakers Bureau; Astra Zeneca: Honoraria, Speakers Bureau; Abbvie: Honoraria, Other: travel, Speakers Bureau. Patti:Novartis: Consultancy; Abbvie: Consultancy, Other: Travel, accommodations; Janssen: Consultancy; Takeda: Consultancy. Reda:Janssen, Abbive, Astra Zeneca, Beigene: Consultancy. Regazzoni:Janssen: Current Employment. Di Candia:Janssen: Current Employment. Mauro:Abbvie, Takeda: Honoraria, Research Funding, Speakers Bureau; Janssen, Astra Zeneca, Beigene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal